what is the one closest drug to soma?
 | |
| Clinical data | |
|---|---|
| Pronunciation | kər-EYE-suh-PROH-dol |
| Trade names | Soma, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682578 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By oral fissure |
| Drug class | Carbamate |
| ATC code |
|
| Legal condition | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | sixty% |
| Metabolism | Liver (CYP2C19-mediated) |
| Metabolites | Meprobamate |
| Onset of action | Rapid (30 minutes[2] [ failed verification ]) |
| Elimination half-life | 2.5 hours [12 hours[nb one]] |
| Excretion | Kidney |
| Identifiers | |
| IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.001.017 |
| Chemical and physical data | |
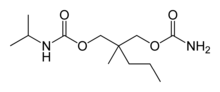
| Formula | C 12 H 24 N ii O 4 |
| Molar mass | 260.334 g·mol−1 |
| 3D model (JSmol) |
|
| SMILES
| |
| InChI
| |
| (verify) | |
Carisoprodol, sold nether the brand name Soma among others, is a medication used for musculoskeletal pain.[iii] Use is but approved for upwardly to iii weeks.[three] Effects generally begin within half an hour and last for up to six hours.[3] It is taken orally.[3]
Common side effects include headache, dizziness, and sleepiness.[3] Serious side effect may include addiction, allergic reactions, and seizures.[3] In people with a sulfa allergy certain formulations may result in bug.[3] Safety during pregnancy and breastfeeding is non clear.[iii] [4] How it works is not articulate.[3] Some of its effects are believed to occur post-obit being converted into meprobamate.[3]
Carisoprodol was canonical for medical use in the United states of america in 1959.[3] Its approving in Europe was withdrawn in 2008.[v] It is available as a generic medication.[iii] In 2017, it was the 255th near ordinarily prescribed medication in the United States, with more than i million prescriptions.[six] [7] In the United States, information technology is a Schedule 4 controlled substance.[3]
Medical uses [edit]

Somadril Comp. – combination muscle relaxant medication containing carisoprodol, paracetamol (acetaminophen), and caffeine
Carisoprodol is meant to be used forth with rest, physical therapy and other mensurate to relax muscles later on strains, sprains and muscle injuries.[8] It comes in tablet format and is taken by the oral fissure three times a day and before bed.[8]
Side effects [edit]
The usual dose of 350 mg is unlikely to engender prominent side effects other than somnolence, and balmy to pregnant euphoria or dysphoria, but the euphoria is generally short-lived due to the fast metabolism of carisoprodol into meprobamate and other metabolites; the euphoria derived is, according to new research, most likely due to carisoprodol's inherent, potent anxiolytic effects that are far stronger than those produced by its main metabolite, meprobamate, which is often misblamed for the drug-seeking associated with carisoprodol, as carisoprodol itself is responsible for the significantly more intense central nervous system effects than meprobamate lone. Carisoprodol has a unique,[9] qualitatively different from that of meprobamate (Miltown). The medication is well tolerated and without adverse effects in the bulk of patients for whom it is indicated. In some patients, however, and/or early on in therapy, carisoprodol can have the full spectrum of sedative side effects and can impair the patient'southward ability to operate a firearm, motor vehicles, and other machinery of various types, especially when taken with medications containing alcohol, in which case an alternative medication would exist considered. The intensity of the side furnishings of carisoprodol tends to lessen every bit therapy continues, equally is the case with many other drugs. Other side effects include: dizziness, clumsiness, headache, fast middle rate, upset tum, airsickness and peel rash.[viii]
The interaction of carisoprodol with essentially all opioids, and other centrally acting analgesics, but peculiarly codeine, those of the codeine-derived subgroup of the semisynthetic class (ethylmorphine, dihydrocodeine, hydrocodone, oxycodone, nicocodeine, benzylmorphine, the diverse acetylated codeine derivatives including acetyldihydrocodeine, dihydroisocodeine, nicodicodeine and others) which allows the use of a smaller dose of the opioid to accept a given effect, is useful in full general and peculiarly where skeletal muscle injury and/or spasm is a large part of the problem. The potentiation effect is likewise useful in other pain situations and is besides especially useful with opioids of the open up-concatenation form, such equally methadone, levomethadone, ketobemidone, phenadoxone and others. In recreational drug users, deaths have resulted from combining doses of hydrocodone and carisoprodol. Some other danger of misuse of carisoprodol and opiates is the potential to aspirate while unconscious.[ commendation needed ]
Meprobamate and other musculus-relaxing drugs oft were subjects of misuse in the 1950s and 60s.[ten] [11] Overdose cases were reported as early every bit 1957, and take been reported on several occasions since so.[12] [thirteen] [xiv] [15] [16] [17] [18]
Carisoprodol is metabolized by the liver and excreted by the kidneys and so this drug must exist used with caution with patients that have impaired hepatic or renal office.[19] Because of potential for more severe side effects, this drug is on the list to avoid for elderly people.[twenty]
Withdrawal [edit]
Carisoprodol, meprobamate, and related drugs such equally tybamate, have the potential to produce physical dependence of the barbiturate type following periods of prolonged employ. Withdrawal of the drug later on extensive apply may require hospitalization in medically compromised patients. In severe cases the withdrawal can mimic the symptoms of alcohol withdrawal including the potentially lethal status epilepticus.
Psychological dependence has also been linked to carisoprodol use[21] although this is much less severe than with meprobamate itself (presumably due to the slower onset of effects). Psychological dependence is more common in those who use carisoprodol non-medically and those who have a history of substance use (particularly sedatives or alcohol). It may reach clinical significance before physiological tolerance and dependence have occurred and (as with benzodiazepines) has been demonstrated to persist to varying degrees of severity for months or years after discontinuation.
Discontinuation of carisoprodol, as with all GABA-ergics, can result in cognitive changes which persist for weeks, months, or rarely even years including greatly increased feet and depression, social withdrawal, pilus-trigger agitation/aggression, chronic indisposition, new or aggravated (often illogical) phobias, reduced IQ, short term and long-term memory loss, and dozens of other sequelae.[22] The effects, severity, and duration appear to be slightly dose-dependent but are mainly determined by the patients pattern of utilise (taken equally prescribed, taken in bulk doses, mixed with other drugs, a combination of the above, etc.), genetic predisposition to substance use, and a history of substance use all increase the patients run a risk of persistent discontinuation syndrome symptoms.
Treatment for physical withdrawal generally involves switching the patient to a long-interim benzodiazepine such as diazepam or clonazepam then slowly titrating them off the replacement drug completely at a rate which is both reasonably comfortable for the patient but rapid plenty for the managing physician to consider the charge per unit of progress acceptable (overly rapid dose reduction profoundly increases the risk of patient non-compliance such as the use of illicitly obtained culling sedatives and/or booze). Psychotherapy and cognitive behavioral therapy take demonstrated moderate success in reducing the rebound anxiety which results upon carisoprodol discontinuation but only when combined with regular and active attendance to a substance employ support group.[ commendation needed ]
Carisoprodol withdrawal tin can exist life-threatening (especially in high dose users and those who attempt to quit "cold turkey"). Medical supervision is recommended, with gradual reduction of dose of carisoprodol or a substituted medication, typical of other depressant drugs.
Non-medical employ [edit]
Combining a muscle relaxant like carisoprodol with opioids and benzodiazepines is referred to as "The Holy Trinity" every bit information technology has been reported to increase the power of the "loftier".[23]
Recreational users of carisoprodol commonly seek its potentially heavy sedating, relaxant, and anxiolytic effects.[24] Also, considering of its potentiating effects on narcotics, it is often used in conjunction with many opioid drugs. Too information technology is not detected on standard drug testing screens. On 26 March 2010 the DEA issued a Observe of Hearing on proposed dominion making in respect to the placement of carisoprodol in schedule IV of the Controlled Substances Act.[25] The DEA concluded upward classifying it under schedule Four.[26] Carisoprodol is sometimes mixed with appointment rape drugs.[27]
Many overdoses take resulted from recreational users combining these drugs to combine their private effects without being aware of the enzyme-induction induced potentiation.[ medical commendation needed ]
Overdose [edit]
As with other GABAergic drugs, combination with other GABAergic drugs, including alcohol, as well as with sedatives in general, possess a pregnant risk to the user in the form of overdose. Overdose symptoms are similar to those of other GABAergics including excessive sedation and unresponsiveness to stimuli, severe ataxia, amnesia, defoliation, agitation, intoxication and inappropriate (potentially violent) behavior. Severe overdoses may present with respiratory depression (and subsequent pulmonary aspiration), coma, and decease.[ citation needed ]
Carisoprodol is not detected on all toxicology tests which may delay diagnosis of overdose. Overdose symptoms in combination with opiates are similar merely are distinguished by the presentation of normal or pinpoint pupils, which are generally unresponsive to low-cal. Carisoprodol (as with its metabolite meprobamate) is particularly dangerous in combination with alcohol. Flumazenil (the benzodiazepine antidote) is not constructive in the management of carisoprodol overdose equally carisoprodol acts at the barbiturate bounden site. Treatment mirrors that of barbiturate overdoses and is generally supportive, including the administration of mechanical respiration and pressors every bit implicated (and in rare cases, bemegride). Total amnesia of the experience is not uncommon following recovery.[ citation needed ]
In 2014 actress Skye McCole Bartusiak died of an overdose due to the combined effects of carisoprodol, hydrocodone and difluoroethane.[28]
Pharmacology [edit]
Pharmacodynamics [edit]
Carisoprodol, has a chemical structure similar to Glutamate, a neurotransmitter, and dimethylglycine. Upon analysis, this pharmacological amanuensis seems to exist an agonist of the NMDA receptor, with an unknown [Km]. Since excess Glutamate causes excitotoxicity and neuronal apoptosis, Carisoprodol overdose may also pb to NMDA related toxicity, thus inducing seizures at loftier doses, and muscle relaxation upon assistants.
Carisoprodol's structural similarity to Meprobamate indicates GABAergic activity, including GABA A agonism, similar to the mechanism of benzodiazepines.[29] This will allow for further muscle relaxation and anxiety reduction. Therefore, Carisoprodol, at low to moderate dosages, may exist clinically indicated for absent-minded seizures, yet exacerbate Tonic-clonic seizures.
Pharmacokinetics [edit]
Carisoprodol has a rapid, 30-minute onset of activeness, with the aforementioned furnishings lasting about two to half-dozen hours. Information technology is metabolized in the liver via the cytochrome P450 oxidase isozyme CYP2C19, excreted by the kidneys and has about an viii-60 minutes half-life. In patients with low levels of CYP2C19 (poor metabolizers), standard doses tin can lead to increased concentrations of carisoprodol (up-to a 4-fold increase).[30] A considerable proportion of carisoprodol is metabolized to meprobamate, which is a known addictive substance; this could account for the addictive potential of carisoprodol (meprobamate levels reach higher meridian plasma levels than carisoprodol itself following administration). Equally mentioned above, carisoprodol appears to accept stiff anxiolytic furnishings on its own; however, a big part of its furnishings also come from the fact that it is metabolized into meprobamate: at to the lowest degree a 25% of the carisoprodol administered will be transformed into meprobamate which means that meprobamate is 3.25× stronger than carisoprodol (although this rate varies from person to person according to their levels of CYP2C19 enzymes in their livers with some people having considerably higher leverls) or, in other words, 200 mg of meprobamate (which is the lowest standard dose) is equivalent to 650 mg of carisoprodol.[ii] As such, meprobamate is believed to play a pregnant role in the furnishings of carisoprodol and meprobamate's long half-life results in bioaccumulation following extended periods of carisoprodol assistants.
It is slightly soluble in water and freely soluble in ethanol, chloroform and acetone. The drug'due south solubility is practically independent of pH.
History [edit]
On 1 June 1959, several American pharmacologists convened at Wayne State University in Detroit, Michigan to hash out a new drug. The drug, originally thought to take clarified properties, was found to take cardinal musculus-relaxing properties.[31] It had been developed by Frank Berger at Wallace Laboratories and was named carisoprodol.
Carisoprodol was a modification of meprobamate, intended to have meliorate muscle relaxing properties, less potential for addiction, and a lower risk of overdose.[32] The substitution of 1 hydrogen cantlet with an isopropyl group on one of the carbamyl nitrogens was intended to yield a molecule with new pharmacological properties.
Usage and legal status [edit]
Norway [edit]
Reports from Norway take shown carisoprodol has addictive potential[33] as a prodrug of meprobamate and/or potentiator of hydrocodone, oxycodone, codeine, and similar drugs. In May 2008 it was taken off the market in Norway.[34]
European Wedlock [edit]
In the European union, the European Medicines Agency issued a release recommending member states append marketing dominance for this production in the treatment of astute (not chronic) back pain.[35]
Equally of November 2007, carisoprodol has been taken off the market in Sweden due to problems with dependence and side effects. The agency overseeing pharmaceuticals considered other drugs used with the same indications as carisoprodol to accept the same or better effects without the risks of the drug.[36]
United States [edit]
Until 12 December 2011, when the administrator of the Drug Enforcement Administration (DEA) issued the final ruling placing the substance carisoprodol into Schedule IV of the Controlled Substances Act (CSA), carisoprodol was non a controlled substance. The placement of carisoprodol into Schedule 4 was constructive eleven Jan 2012.[37]
Carisoprodol is available generically as 350 mg and, more than recently, 250 mg tablets. Compounded tablets with acetaminophen and codeine are besides available.[38]
Canada [edit]
Federally, carisoprodol is a prescription drug (Schedule I, sub-schedule F1).[39] Provincial regulations vary.[forty] Information technology is no longer readily available.[ medical citation needed ]
Indonesia [edit]
- In September 2013, carisoprodol was taken off the market due to problems with diversion, dependence and side effects.
- In September 2017, one child died and 50 suffered seizures when PCC, which stands for "Paracetamol Caffeine Carisoprodol" was mixed (probably illicit) into children's drinks in elementary and junior high schools in Kendari.[41]
Notes [edit]
- ^ At least 25% of the carisoprodol in the torso is transformed by the liver into meprobamate, its principal agile metabolite, which in turn has a half-life of 10 hours.[2]
References [edit]
- ^ "Carisoprodol". drugs.com . Retrieved sixteen April 2017.
- ^ a b c Carrasco A (thirteen September 2019). "Letra C (Carisoprodol)". In Carrasco Ruiz MA, Chavez Pulido X, Morales E (eds.). Diccionario de Especialidades Farmaceúticas PLM. Diccion (in Spanish). Vol. I (65th ed.). Mexico City: PLM Latinoamérica. p. 222. ISBN978-607-625-072-3 . Retrieved 13 June 2021.
- ^ a b c d eastward f g h i j thou fifty thou "Carisoprodol Monograph for Professionals". Drugs.com. American Social club of Wellness-System Pharmacists. Retrieved 8 Apr 2019.
- ^ "DailyMed - carisoprodol tablet". dailymed.nlm.nih.gov . Retrieved 8 Apr 2019.
- ^ "Carisoprodol". European Medicines Agency. 15 November 2007. Retrieved eight Apr 2019.
- ^ "The Tiptop 300 of 2020". ClinCalc . Retrieved 11 April 2020.
- ^ "Carisoprodol - Drug Usage Statistics". ClinCalc . Retrieved 11 Apr 2020.
- ^ a b c "Carisoprodol". MedlinePlus. National Library of Medicine. Retrieved 6 May 2019.
- ^ mechanism of action
- ^ Kamin I, Shaskan DA (June 1959). "Death due to massive overdose of meprobamate". The American Journal of Psychiatry. 115 (12): 1123–4. doi:10.1176/ajp.115.12.1123-a. PMID 13649976.
- ^ Hollister LE (1983). "The pre-benzodiazepine era". Journal of Psychoactive Drugs. 15 (1–2): 9–13. doi:10.1080/02791072.1983.10472117. PMID 6350551.
- ^ Gaillard Y, Billault F, Pépin Chiliad (May 1997). "Meprobamate overdosage: a continuing problem. Sensitive GC-MS quantitation after solid phase extraction in 19 fatal cases". Forensic Science International. 86 (3): 173–80. doi:x.1016/S0379-0738(97)02128-2. PMID 9180026.
- ^ Allen MD, Greenblatt DJ, Noel BJ (Dec 1977). "Meprobamate overdosage: a continuing problem". Clinical Toxicology. 11 (5): 501–15. doi:ten.3109/15563657708988216. PMID 608316.
- ^ Kintz P, Tracqui A, Mangin P, Lugnier AA (June 1988). "Fatal meprobamate self-poisoning". The American Journal of Forensic Medicine and Pathology. 9 (ii): 139–40. doi:10.1097/00000433-198806000-00009. PMID 3381792.
- ^ Eeckhout Eastward, Huyghens L, Loef B, Maes 5, Sennesael J (1988). "Meprobamate poisoning, hypotension and the Swan-Ganz catheter". Intensive Care Medicine. fourteen (4): 437–8. doi:10.1007/BF00262904. PMID 3403779. S2CID 2784867.
- ^ Lhoste F, Lemaire F, Rapin M (April 1977). "Handling of hypotension in meprobamate poisoning". The New England Journal of Medicine. 296 (17): 1004. doi:ten.1056/NEJM197704282961717. PMID 846530.
- ^ Bedson HS (Feb 1959). "Coma due to meprobamate intoxication; report of a case confirmed by chemic analysis". Lancet. i (7067): 288–90. doi:10.1016/S0140-6736(59)90209-0. PMID 13632000.
- ^ Blumberg AG, Rosett HL, Dobrow A (September 1959). "Severe hypotensive reactions post-obit meprobamate overdosage". Register of Internal Medicine. 51 (3): 607–12. doi:x.7326/0003-4819-51-iii-607. PMID 13801701.
- ^ "CARISOPRODOL". TOXNET. National Library of Medicine. Retrieved vi May 2019.
- ^ NCQA's HEDIS Measure: Use of High Gamble Medications in the Elderly Archived 1 February 2010 at the Wayback Machine
- ^ "What is Carisoprodol used for?". Hurting o Soma medicines. 19 March 2021. Retrieved 29 Apr 2021.
- ^ Barker MJ, Greenwood KM, Jackson Yard, Crowe SF (April 2004). "Persistence of cerebral effects subsequently withdrawal from long-term benzodiazepine utilise: a meta-analysis". Archives of Clinical Neuropsychology. 19 (iii): 437–54. doi:10.1016/S0887-6177(03)00096-nine. PMID 15033227.
- ^ Horsfall JT, Sprague JE (February 2017). "The Pharmacology and Toxicology of the 'Holy Trinity'". Bones & Clinical Pharmacology & Toxicology. 120 (2): 115–119. doi:x.1111/bcpt.12655. PMID 27550152. S2CID 25909460.
- ^ "DEA Drugs & Chemicals of Business concern "Carisoprodol"". Archived from the original on 17 April 2011. Retrieved 29 April 2011.
- ^ "Schedules of Controlled Substances: Placement of Carisoprodol Into Schedule 4; Announcement of Hearing". Archived from the original on 15 July 2011. Retrieved 19 Apr 2010.
- ^ "Carisoprodol" (PDF). Drug Enforcement Administration, Diversion Control Division, Drug & Chemical Evaluation Section. U.S. Department of Justice. December 2019.
- ^ Madea B, Musshoff F (May 2009). "Knock-out drugs: their prevalence, modes of action, and means of detection". Deutsches Ärzteblatt International. 106 (20): 341–seven. doi:10.3238/arztebl.2009.0341. PMC2689633. PMID 19547737.
- ^ Duke A (22 July 2014). "'Patriot' actress Skye McCole Bartusiak dead at 21". CNN Entertainment . Retrieved 24 Feb 2019.
- ^ Conermann, Till; Christian, Desirae (2022), "Carisoprodol", StatPearls, Treasure Isle (FL): StatPearls Publishing, PMID 31971718, retrieved 23 February 2022
- ^ Dean, Laura (four Apr 2017). "Carisoprodol Therapy and CYP2C19 Genotype". Medical Genetics Summaries.
- ^ Miller JG, ed. The pharmacology and clinical usefulness of carisoprodol. Detroit:Wayne State University; 1959.
- ^ Berger FM, Kletzkin Thousand, Ludwig BJ, Margolin S (March 1960). "The history, chemistry, and pharmacology of carisoprodol". Annals of the New York Academy of Sciences. 86: 90–107. doi:10.1111/j.1749-6632.1960.tb42792.x. PMID 13799302. S2CID 11909344.
- ^ Bramness JG, Furu K, Engeland A, Skurtveit S (Baronial 2007). "Carisoprodol employ and corruption in Kingdom of norway: a pharmacoepidemiological report". British Journal of Clinical Pharmacology. 64 (2): 210–eight. doi:10.1111/j.1365-2125.2007.02847.ten. PMC2000626. PMID 17298482.
- ^ "Somadril trekkes fra markedet" [Somadril is withdrawn from the market]. Norwegian Medicines Agency (in Norwegian). twenty Apr 2008. Archived from the original on 16 July 2011. Retrieved 12 March 2010.
- ^ "Carisprodol printing release" (PDF). EMEA. Archived from the original (PDF) on 18 July 2009. Retrieved 12 May 2008.
- ^ "Marknadsföringen av Somadril och Somadril comp rekommenderas upphöra tillfälligt" [Marketing of Somadril and Somadril is recommended to terminate temporarily] (in Swedish). xvi November 2007. Archived from the original on 23 July 2014. Retrieved 9 May 2009.
- ^ US Section of Justice (2011). "Schedules of Controlled Substances: Placement of Carisoprodol into Schedule 4" (PDF). Federal Annals. 76 (238): 77330–77360. Retrieved 1 February 2012.
- ^ "High Toll, No Benefit – The Rheumatologist". the-rheumatologist.org. Archived from the original on 7 May 2015. Retrieved 31 Baronial 2017.
- ^ "NAPRA – Search National Drug Schedule". National Association of Pharmacy Regulatory Authorities. 2009. Archived from the original (ASP) on 1 February 2014. Retrieved vii January 2014.
- ^ For British Columbia, see library.bcpharmacists.org/D-Legislation_Standards Archived 17 Dec 2013 at the Wayback Automobile
- ^ "One Schoolchild Dies, More Than l Suffer Seizures After Consuming Pills in Southeast Sulawesi". Jakarta Globe. xiv September 2017.
Farther reading [edit]
- Dean L (2017). "Carisoprodol Therapy and CYP2C19 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Middle for Biotechnology Information (NCBI). PMID 28520377. Bookshelf ID: NBK425390.
External links [edit]
- "Carisoprodol". Drug Information Portal. U.Due south. National Library of Medicine.
Source: https://en.wikipedia.org/wiki/Carisoprodol
0 Response to "what is the one closest drug to soma?"
Post a Comment